Research
Dedicating to finding a pattern of facts.

Chen’s group is motivated to develop methods to measure a variety of materials such as DNA, protein, polymer, ceramic, semiconductor, and metals with specific interests in surface and interfacial chemistry. A particular focus is on spectroscopy and microscopy characterization of these materials to understand their performance in biosensing, nanodevices, and solar cells. His group is also interested in developing computational software packages for analyzing data obtained from these measurements, especially on reaction kinetics.
Example major contributions of the group include reaction kinetics such as the theory of single-molecule adsorption kinetics, quantum dot surface reaction mechanism, click chemistry kinetic model, mixed halide perovskite phase separation kinetics; COVID-19 kinetics; biophysics of dye-DNA interactions; and surface functionalization strategies. For example, Chen proposed a reaction kinetic model for the adsorption of diffusion molecules from the bulk solution to target molecules that are immobilized on a surface, a standard scenario for almost any heterogeneous reaction. This has been a challenge for >200 years with significant progress made by many exceptional scientists. Chen proposes a theory of fraction reaction kinetics that is very nonintuitive but supported by the experimental results in his 2022 AIP paper for this process, and 2022 JPCA paper for adsorption in the bulk solution.
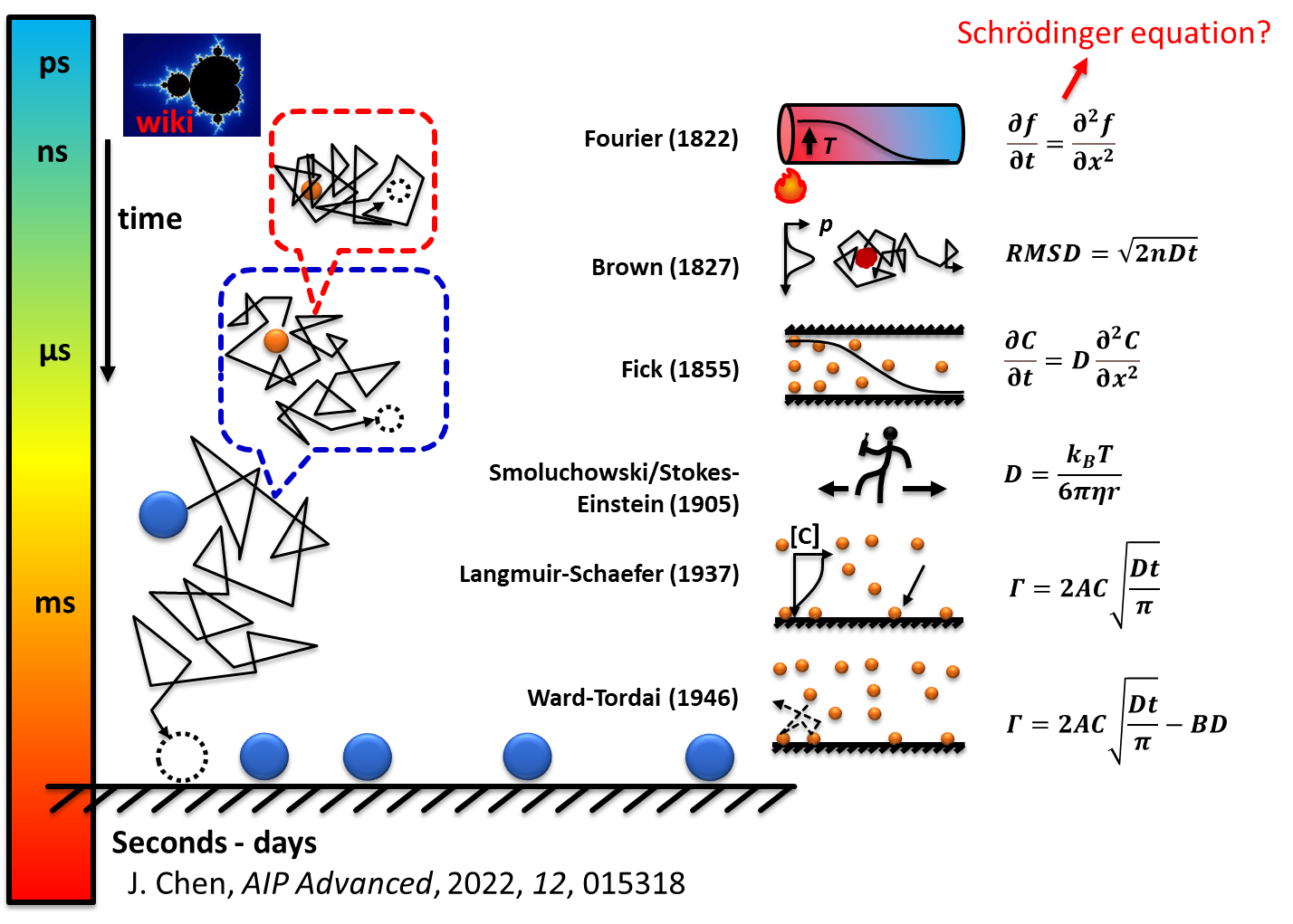
This fraction reaction order is because molecular displacement of molecules under diffusion is non-linearly dependent on time which is not intuitive to the linear correlation between moving objects we observe in our daily life. A concept of adsorption cross-section is proposed for the first time which could be the only free parameter in a given adsorption system in calculating the adsorption rate. This theory opens windows to many fields such as biology, environment, industrial reaction kinetics, and batteries.

As experimental physical chemists, the overall goal of our research is to develop and apply techniques and methods to understand the complex behavior of molecules and nanoparticles at surfaces and interfaces, leading to the development of new materials and technologies that will benefit our lives.
Single Molecule Kinetics
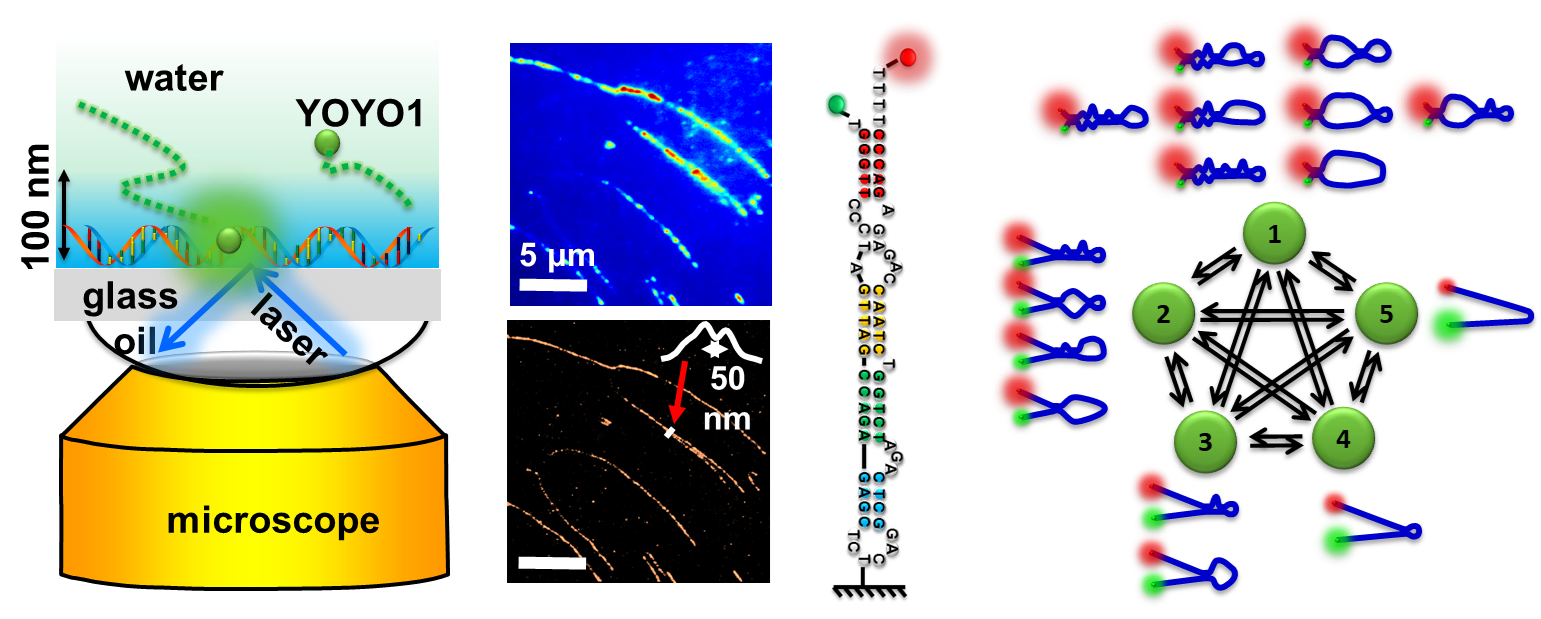
We develop molecular probes for single-DNA imaging. We synthesize various nanoparticles and quantum dots for photoluminescence measurement and nanodevice fabrication. We use fluorescence microscopy to measure single-molecule diffusion/binding kinetics, single-molecule FRET kinetics, and single-particle fluorescence kinetics. We use statistical methods to analyze these data. The figure shows examples of using Monte Carlo simulations for the adsorption of molecules on a surface with 1D and 3D models. We are developing MATLAB codes for data analysis, fitting, and simulations on various kinetic systems, such as smFRET, reaction kinetics, and diffusion.
Materials Science
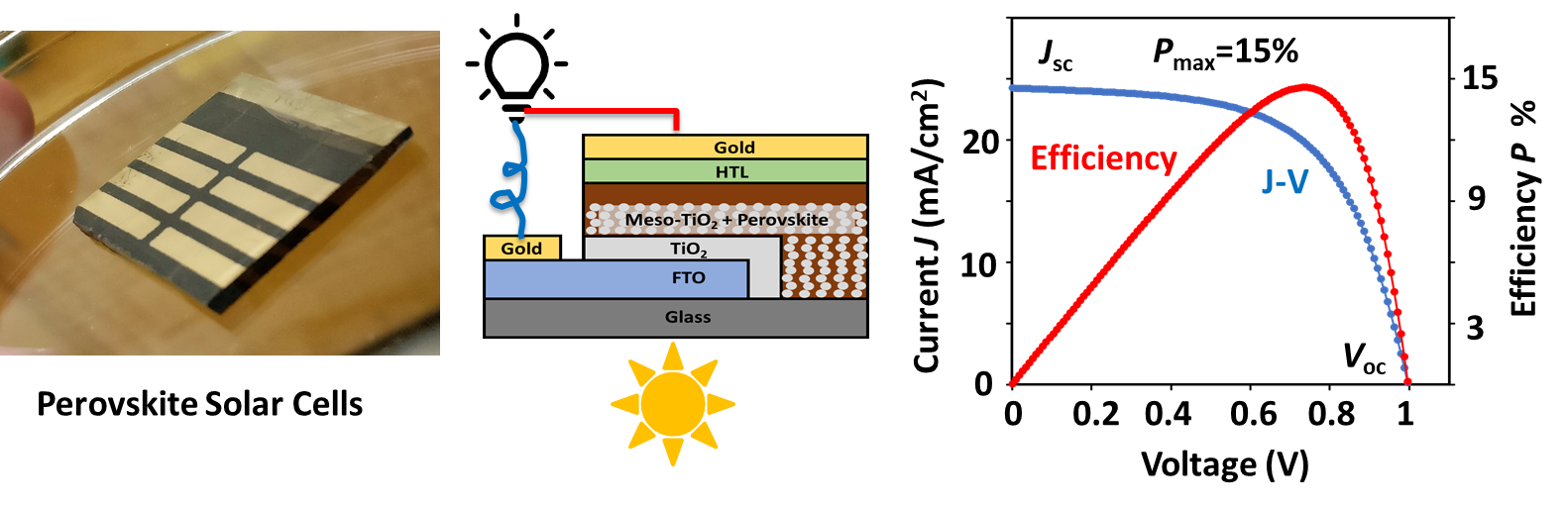

Our group is making thin-film solar cells and characterizing their interfacial chemical and electrochemical properties. The example figure shows a perovskite solar cell (PSC) made and tested in 2018 in our lab with >15% solar efficiency at AM 1.5G standard sunlight illumination. PSC is a type of promising next-generation solar cell with a world record solar efficiency >26% 2024.
We also synthesize perovskite quantum dots and study their photoluminescent properties, especially under different conditions and passivations. These materials are promising to make light-emitting diodes (LEDs), lasers, and display screens.
Collaborators

|
National Chiao Tung University, Taiwan |

|
Loyola University, Chicago, IL |

|
Ohio University, Athens, OH |

|
Ohio University, Athens, OH |

|
University of Cincinnati, OH |

|
Ohio University, Athens, OH |
|
Ohio University, Athens, OH |

|
Ohio University, Athens, OH |

|
Ohio University, Athens, OH
Department of Electrical Engineering and Computer Science
|
Internal Fund (Ohio University)
| 2023 | NQPI Research Challenge Award, internal grant |
| 2022 | OU 1804 Award, Acquisition of an FT-IR Spectrophotometer for Teaching and Research |
| 2019 | OU 1804 Award, Spectrometer for Education and Research in Forensic Chemistry |
| 2019 | NQPI Research Challenge Award, internal grant |
| 2018 | HTC Undergraduate Student Research Apprenticeship Fund |
| 2017 | PACE Undergraduate Student Research Fund |
| 2017 | NQPI Research Challenge Award |
| 2016 | NQPI Research Challenge Award |
| 2015 | OURC Fund |
| 2015 | HTC Undergraduate Student Research Fund |
| 2014 | Ohio University Startup Fund |
|

|
|
|
Facility and in-house instruments
Sharing space with the physical chemistry division, occupying about a third of the 4000 ft^2 lab space and 6 faculty and student offices in new chemistry building east wing of 3rd floor.
- Wet lab : ~800 ft^2 equipted with benchtops, cabins, and three fume hoods, a laminar flow clean hood, water, sink, and an ultrapure water system.
- Laser lab: ~400 ft^2 equipted with hang-on power rack, storage, and light control system.
- PI and Student Offices across the hallway.
- Have access to chemistry machine shop, chemistry stockroom, physics machine shop, and electronic shop.
- Have access to NQPI and Ohio University shared equipment and facility.
In-house instruments:
- A super-resolution spectro-microscope is ready for this project. More specifically, the microscope (Nikon TiU) is equipped with four lasers (405, 473, 532, and 635 nm), a 1.49 NA, 100x, oil-immersion objective (Nikon CFI Apo), a 20x Nikon objective, filter sets, eyepieces, and a -100 oC cooled EMCCD detector (Andor iXon 897U). The working mode can be switched between total internal reflectance fluorescence (TIRF) and Epi-fluorescence wide-field mode.
- A Raman/AFM/NSOM scanning microscope (AlphaSNOM, Witec GmbH) . This microscope incorporates confocal scanning Raman spectromicroscopy, atomic force microscopy (AFM), and near-field scanning optical microscopy together in one microscope. The microscope has been further modified into a 4-π setup with two objectives focusing on the same plain. Several lasers have been connected to the microscope including CW lasers at 405 nm, 532 nm, and 980 nm, and a pulse laser at 532 nm. A high-resolution spectrometer (detector -100 oC) and a fast single-photon avalanche photodiode (APD) detector have been attached. A temperature-control microscope stage is equipped with this microscope. This microscope is obtained from Dr. Richardson, a recently retired colleague of the PI. This instrument is now shared in the physical chemistry division with Dr. Cimatu for teaching and research.
- An atomic force microscope (MFP 3D AFM, Asylum Research shared with Dr. Cimatu for both teaching and research).
- A differential scanning calorimeter (DSC shared with Dr. Cimatu for both teaching and research).
- A fluorometer (Horiba shared with Dr. Cimatu for teaching and research)
- MATLAB codes for data analysis of single-molecule and single-particle photoluminescence, MATLAB codes for ultrafast TA data global fitting, and PL lifetime fitting have been developed and tested in several publications.
- A Dynamic light scattering spectrometer (DynaPro).
- A VASE Ellipsometer (VASE HS-190, tunable wavelengths, also shared for teaching).
- A single-photon counting spectrofluorometer (the best time resolution is 40 ps).
- A gold sputter (Denton Vacuum) and a metal thermal evaporator.
- A Plasma cleaner (Harrick Plasma).
- Two spin coaters (MTI and Ossila).
- A solar simulator (Abet).
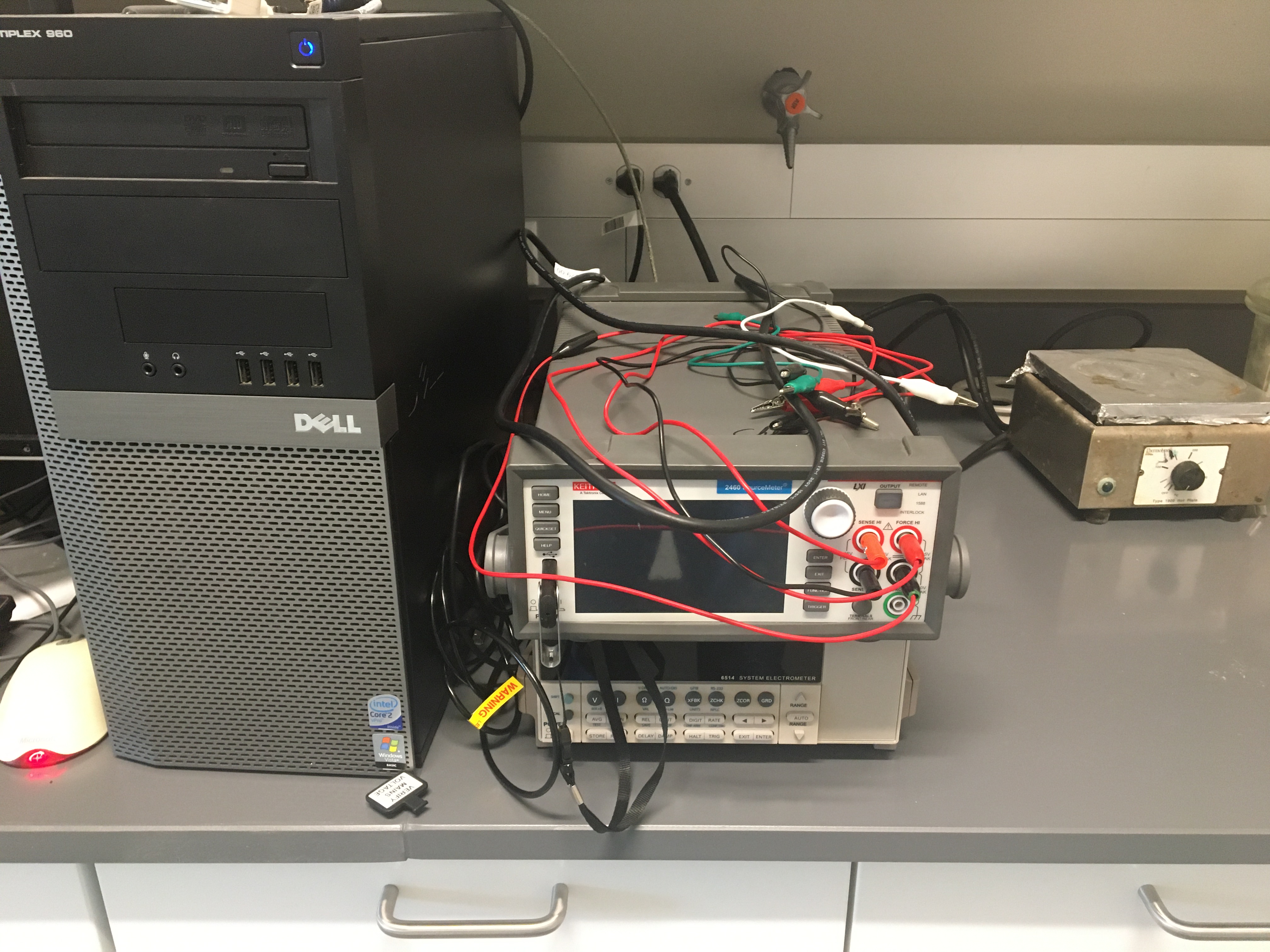
- A Keithley 2460 power source meter.
- A Keithley 6514 electrometer.
- A regular fluorescence microscope.
- A UV-Vis spectrometer.
- Other basic lab equipment such as balances, centrifuges, ovens, furnaces, refrigerators, safety chemical cabinets, sonicators, a probe sonicator, and a Thermo Barnstead E-Pure water purification system.
|
Home-built super-resolution fluorescence microscopy and spectro-microscopy |
|||
|
|
|||
| Witec Scanning Raman/SNOM/AFM spectroscopic and imaging system | |||

|
|||
| Wavelength Scanning Fluorometer (can do lifetime measurement if we have an additional pulsed laser) | |||

|
|||
| Tunable wavelength ellipsometer | |||

|
|||
| Solar simulator | |||

|
|||
| Cyclic voltameter and potential current meter | |||
|
|||
| Laser cutter | |||

|
|||
| Glovebox with a spin coater inside | |||

|
|||
| Flow hood | |||

|
|||
| Ultrapure water system, and Schlenk line (for orgnaic synthesis outside of glovebox) in the hood | |||

|
|||
| Oven, centrifuge, balance, hoods, and etc. | |||

|


